Vaccines are a miraculous invention of mankind. However, there are always two sides of a coin. Vaccines also may cause adverse reactions; sometimes in a few minutes and rarely, years later. Different reactions/adverse events/AEFIs reported for vaccines indeed should be submitted to concerned regulatory authorities. Some regulatory authorities have defined guidance and formats for reporting vaccine-related adverse events, other than medicine/drug reporting formats while few other regulatory authorities are in process of defining such reports and monitoring process in vaccine safety.
World Health Organization (WHO) is relentlessly working for vaccine safety and monitoring. The global vaccine action plan, immunization surveillance, assessment, and monitoring etc. and other plan of action are available for reference on their website. European medicinal agency (EMA) is also working on this initiative by establishing Vaccine Adverse Event Surveillance & Communication (VAESCO) for recording information related to AEFIs etc. Likewise, US FDA has a VAERS (Vaccine adverse event reporting system) form in order to report vaccine adverse events.
With automation in vaccine safety reporting, PvEdge has ensured to keep pace with electronic reporting for vaccine products. PvEdge is a platform wherein Vaccine safety recording and reporting regulatory submissions are possible.
Why PvEdge?
PvEdge offers below report formats in order to meet the global vaccine adverse event reporting compliance.
Reporting formats integrated in PvEdge:
- Vaccine Adverse Event Safety Reporting Form (VAERS)
- CIOMS Form for Vaccine reporting
- Vaccine XML for submissions to VigiFlow – Global WHO database (as per CDSCO office order of May 2015)
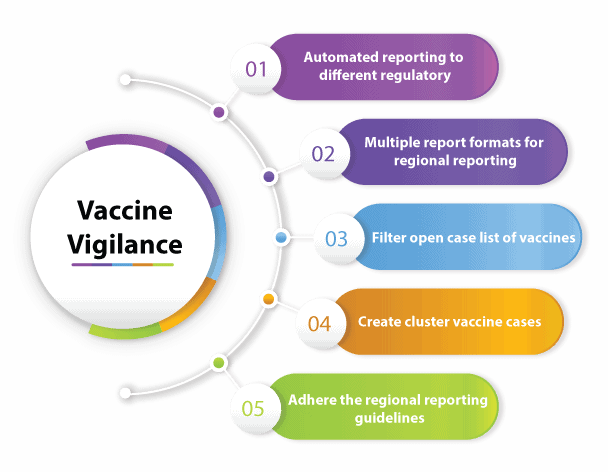
Prominent features of PvEdge’s vaccine module
- Automated reporting to different regulatory authorities
- Different report formats utilized for regional reporting purposes
- Vaccine module prepared to adhere the regional reporting guidelines which is fully complaint with the reporting rules of that particular region
- The possibility of creating cluster vaccine cases
- Filter open case list of vaccines in database
- Providing functionality for Ethics Committees to process SAE’s and generate CDSCO (Central Drugs Standard Control Organization) reports
PvEdge’s submission front module
- Maintaining a track on submissions
- Initial/follow up tracking
- Archiving submitted vaccine reports
- Confirming acknowledgments
- Creating follow-ups alerts
- Full report extraction for vaccine product submissions
- Analyze delayed submissions, if any
