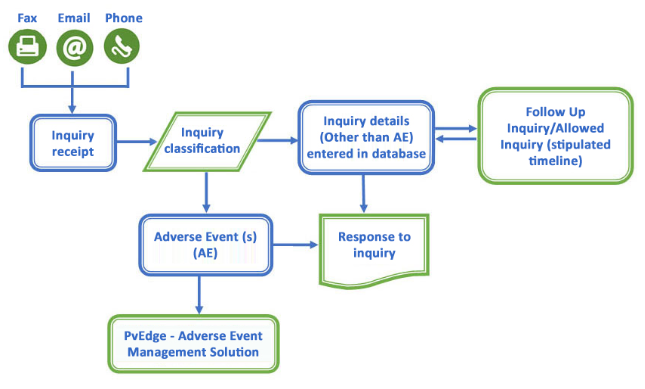
Amidst the complexities of the variety of product information received, it is indeed a challenge to keep track history of the details pertaining to different product inquiries, including the type of product inquiry and in which area should it be appropriately categorized.
In the current scenario, companies want to track all the product relevant information, which we may term as Pro-pharmacovigilance (in support of pharmacovigilance) and deal with all sorts of customer and health care professional issues which have broadly been segregated as inquiries. This includes, but is not limited to:- Product inquiry such as inquiry on labeled information, general query from the consumer, Lack of efficacy information e.g.: Probable emergence of a resistant strain to anti-microbial, allergic reaction of any nature, Misuse/abuse of product.
- Medical information inquiry: Question of healthcare professionals on drug usage technique, query on drug package leaflet etc.
- Product complaints
- Adverse events after drug intake/administration including medication error, overdose, off label use or lack of efficacy
- Other inquiries: Any other general inquiry that does not fall in the above criteria
Product quality complaints (PQCs)
It involves any written, electronic or oral communication that alleges deficiencies related to the identity, quality, durability, reliability, safety, effectiveness, or performance of a medicinal product or device after it is released for distribution to market. This includes all components distributed with the drug, such as packaging, drug containers, delivery system, labelling, and inserts.
Examples include:- Device/Formulation that is damaged or broken
- Physical defect in packaged form of the product
- Bent or blunt needles
- Missing or illegible labeling
- Inability of customer to administer the product
- Product with an unexpected color, appearance, or particles
- User error
Why PrITR?
PrITR is an efficient module to receive, process, evaluate, and record all the Product inquiry, Medical information inquiries, and Product quality complaints (PQC) along with adverse events due to PQCs with regards to global compliance. This product inquiry module generally used to maintain the risk benefit profile of the product.
PrITR can analyze the information received in order to:
- Keep track of the inquiries that were generated.
- Ensure that all the customer and HCP queries are dealt with and documented.
- Know timely, the problems and issues faced for the marketed product.
- Detect a potential adverse event or other drug use problems
- Track product history through recorded information
- Improve the quality of responses generated through quality assurance and training
- Prepare FAQs and material for common and frequent inquiries
- Inquiry can be transferred into PvEdge® or locked/followed up in PrITR if any AE is present
- Dynamic dashboard for product inquiry management
PrITR workflow and functionality